Vehicle engine coolants
Functions and technological differences
How does cooling work in vehicles with a combustion engine? What is the ideal mixing ratio, and can the coolant also be used as an antifreeze agent for the coolant circuit? Why is it important to replace coolant on a regular basis? This article describes the different coolant technologies that are available and the influence the cooling process has on the engine temperature.
In terms of the function and the task, the correct cooling liquid is as important as the engine oil. Incorrect specifications, an unsuitable mix ratio, irregular replacement of the cooling liquid and/or ageing of the cooling liquid lead to corrosion and premature failure of the water pump and other engine parts. The additives in the coolant agent function as ageing stabilisers, corrosion protection, anti-foam agent, detergents and coating material. All additives ensure the proper function and condition of the cooling liquid in accordance with the regulations until the next change.
Some of the most important functions and facts regarding coolant agent are stated below.
 |
ATTENTION |
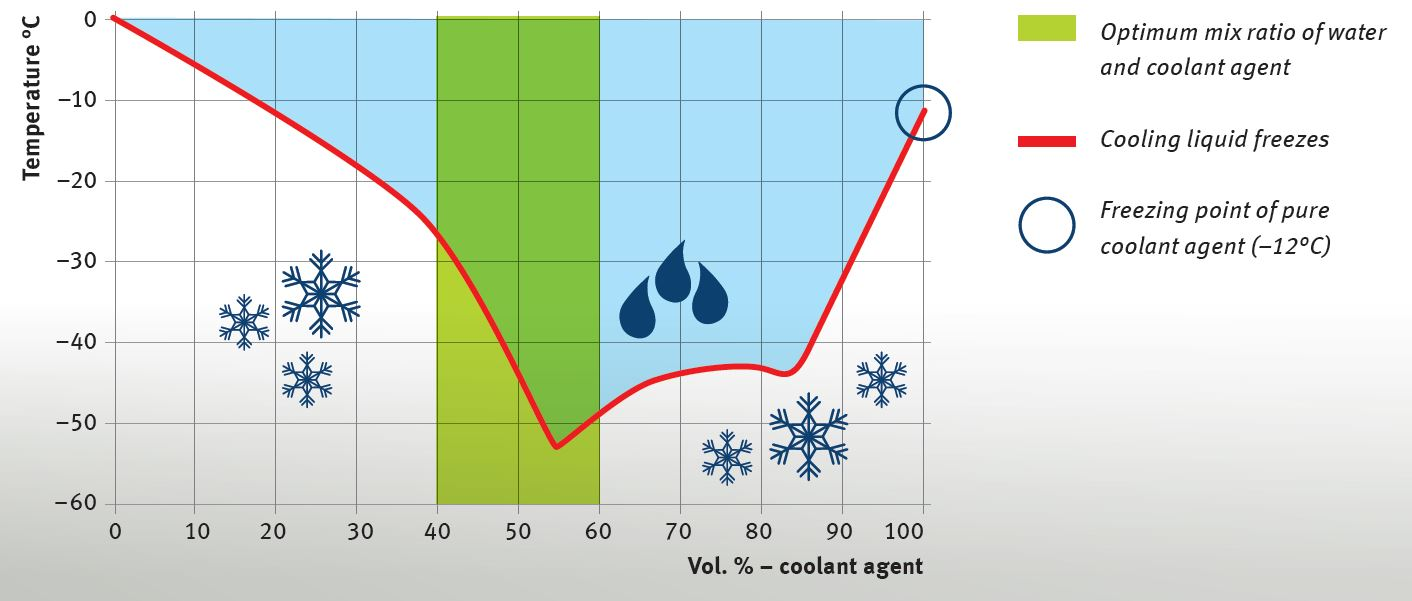
Anti-freeze function of the coolant agent
Undiluted coolant agent must not be used, even in areas in which very low frost temperatures are possible. If the coolant agent is mixed with an insufficient amount of water or if undiluted coolant agent is used, the anti-freeze effect reverses at a certain temperature. This means that the cooling liquid can freeze even at temperatures above –15°C, despite the high concentration of coolant agent.
Thermal absorption capacity of the coolant agent
Engines that are improperly operated using pure water may never reach the correct operating temperature as this means that the cooling system is oversized. For more detailed information on this topic, see Chapter ‚Damage and causes of failure‘.
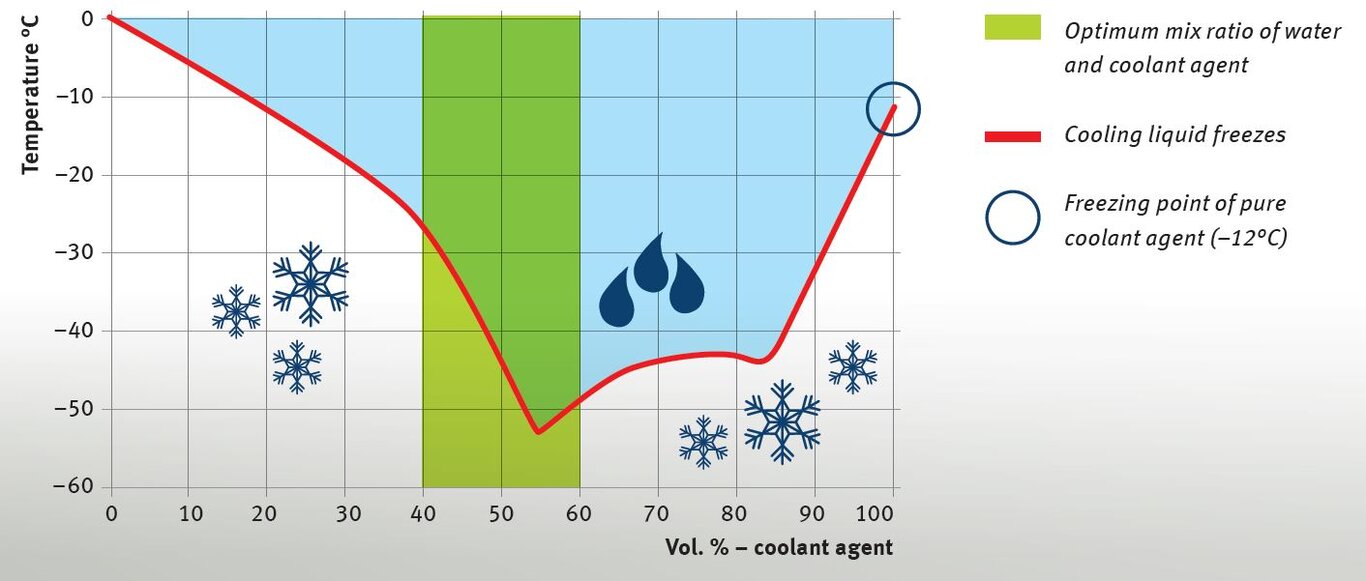
Increasing the boiling point
The graphic shows the vapour pressure curves of certain glycol/water mixtures. The resulting boiling points can be read out at the respective intersections, for example at a operating pressure of 1 bar in the cooling system and with various mix ratios.
* In the case of used vehicles (facility vehicle) that were sold from temperate latitudes to hot climate zones, the size of the vehicle radiator may need to be adjusted in accordance with the manufacturer‘s instructions in order to prevent the engine from overheating. This is something that cannot be effectively prevented by operating the cooling system with pure water and/or with the thermostat removed.
Corrosion protection
Due to a lack of corrosion-inhibiting substances in the cooling liquid, the salts and acids that may be present in the cooling liquid lead to components being chemically attacked (corrosion). In the long term, this deteriorates the engine components. Aluminium corrosion is a common problem in cooling systems in particular.
The oxygen present in the water also oxidises with ferrous materials and pollutes the cooling liquid with solids (rust). The relatively hard rust particles lead to rapid wear on the sliding ring seal of the water pump.
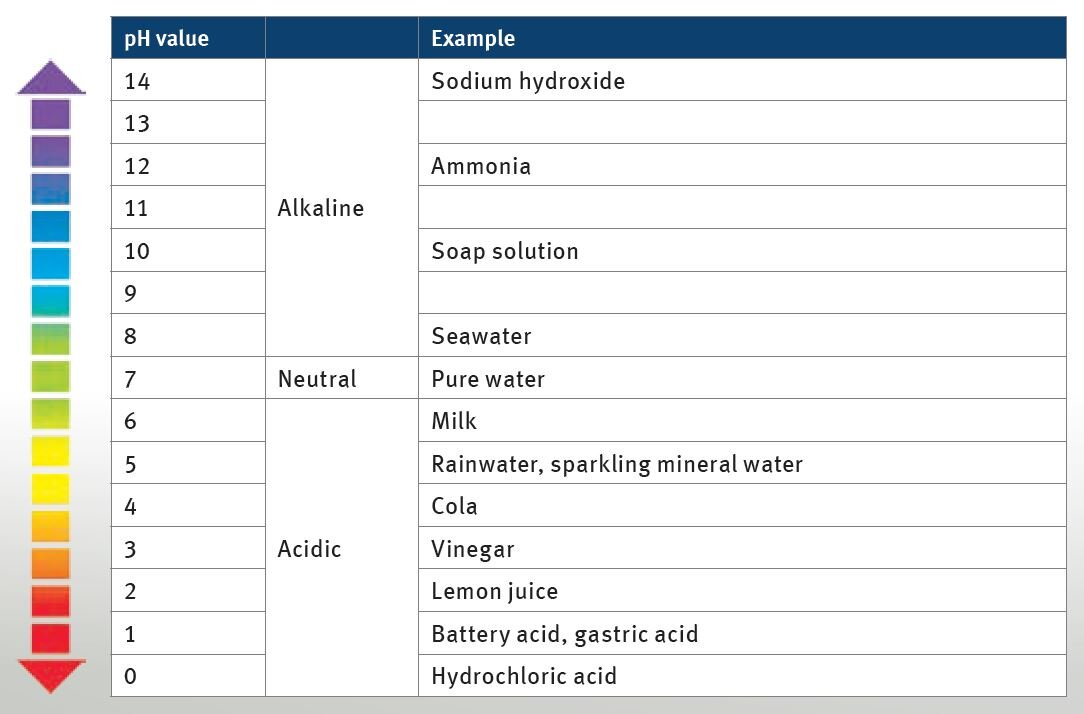
In order to counteract the corrosion, the coolant agent has alkaline properties. The pH value is around 8, providing a buffer effect with regard to acids that enter the cooling system. The buffer effect is decreasing
over time. Salty water, rainwater, deposits of radiator decalcifiers or combustion gases that enter the cooling liquid can move the ratio of acids to bases into the acidic range. Pure (distilled) water has a pH value of 7 and therefore features neutral properties.
The graphic shows the individual pH value range covered by the various example liquids.
Tags
Product groups
This might also interest you


